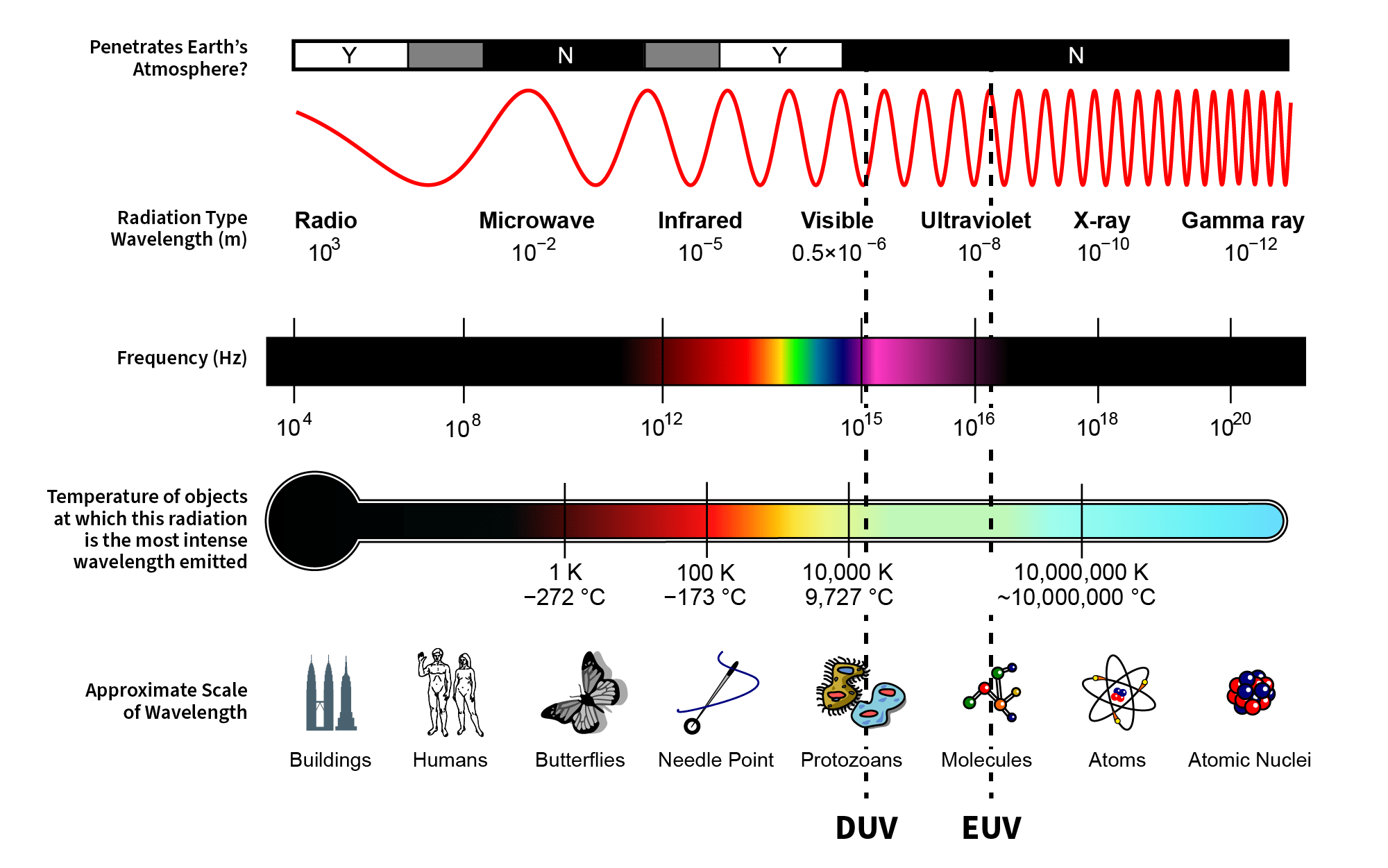
Relationship Between Kinetic Energy And Wavelength - Web the amount of energy in a wave is related to its amplitude and its frequency. Web in relating a particle's energy to its wavelength, two equations are used. Web in classical theory, the photoelectron absorbs electromagnetic energy in a continuous way; Web the energy of each photon is e = hf e = h f, where h is planck’s constant and f is the frequency of the em radiation. To standardize the energy, consider the. Web by the end of this section, you will be able to: The first is the kinetic energy equation: Web the wave can be very long, consisting of many wavelengths. Explain the relationship between the energy of a photon in joules.
Web the energy of each photon is e = hf e = h f, where h is planck’s constant and f is the frequency of the em radiation. Web the amount of energy in a wave is related to its amplitude and its frequency. Web the wave can be very long, consisting of many wavelengths. Web in classical theory, the photoelectron absorbs electromagnetic energy in a continuous way; Web by the end of this section, you will be able to: Web in relating a particle's energy to its wavelength, two equations are used. To standardize the energy, consider the. Explain the relationship between the energy of a photon in joules. The first is the kinetic energy equation: